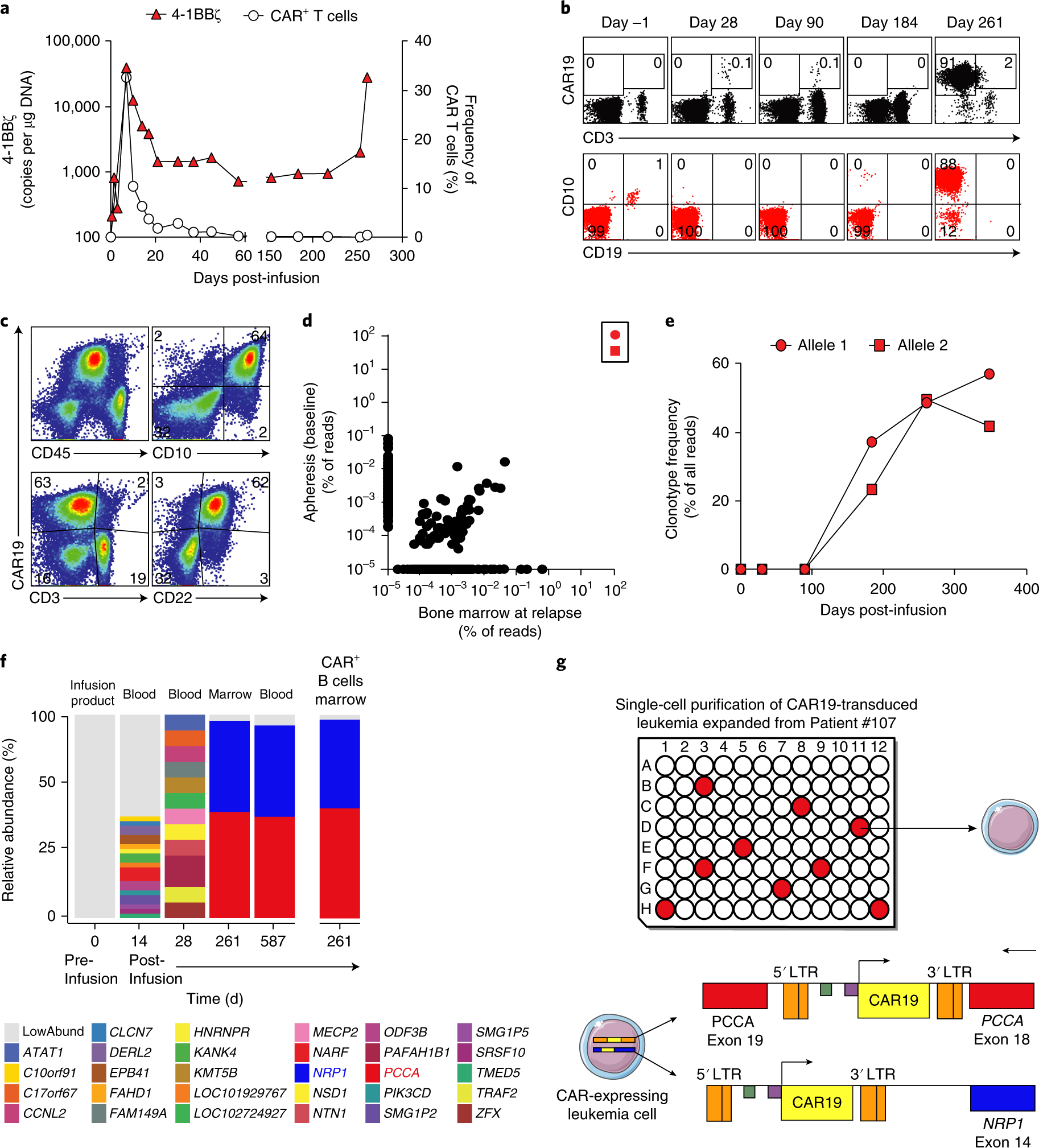
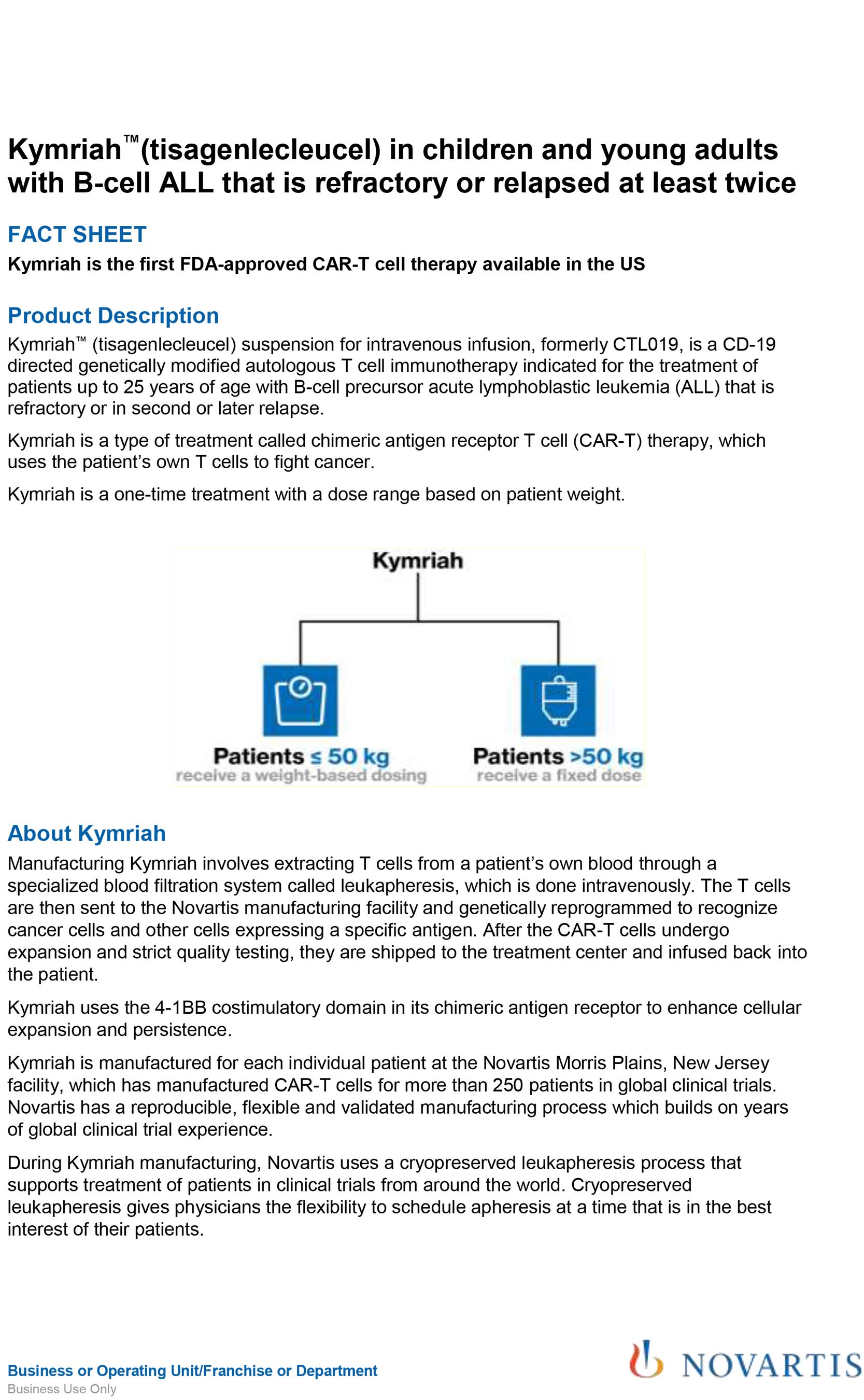
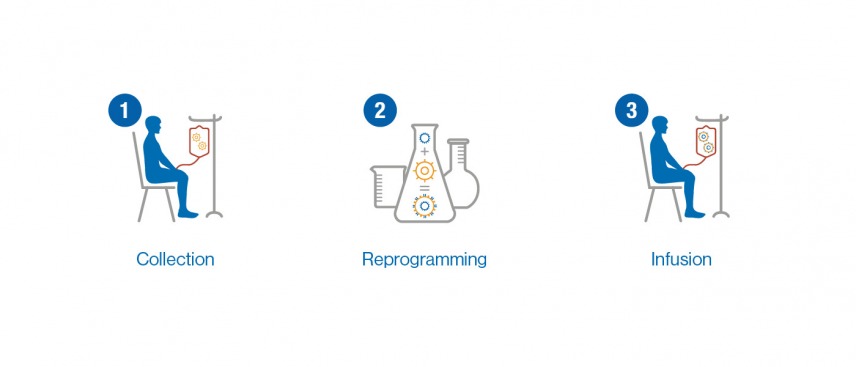
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar
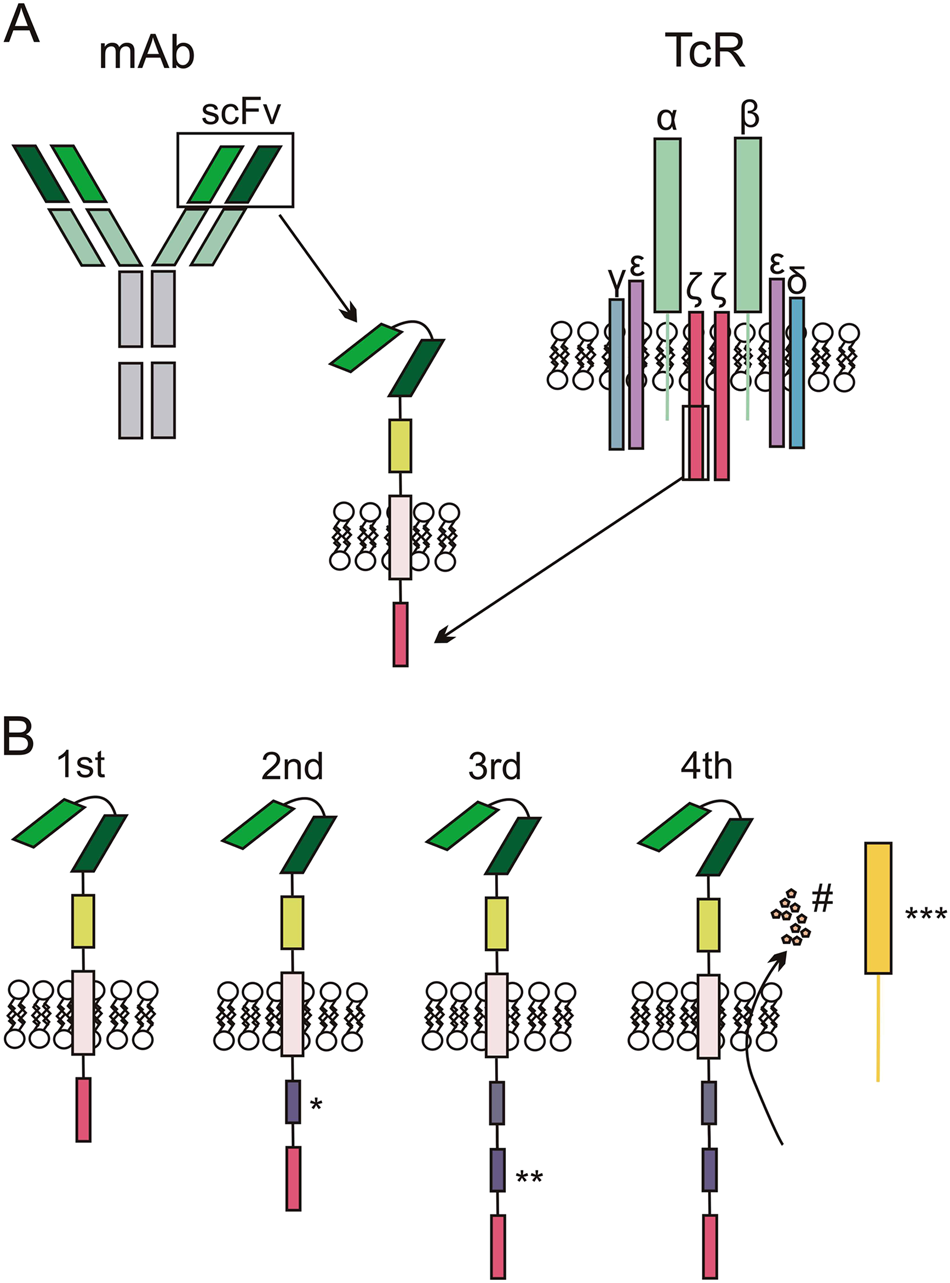
The biological basis and clinical symptoms of CAR-T therapy-associated toxicites | Cell Death & Disease
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar
BLA 125646 Tisagenlecleucel 1 FDA Briefing Document Oncologic Drugs Advisory Committee Meeting BLA 125646 Tisagenlecleucel Novar
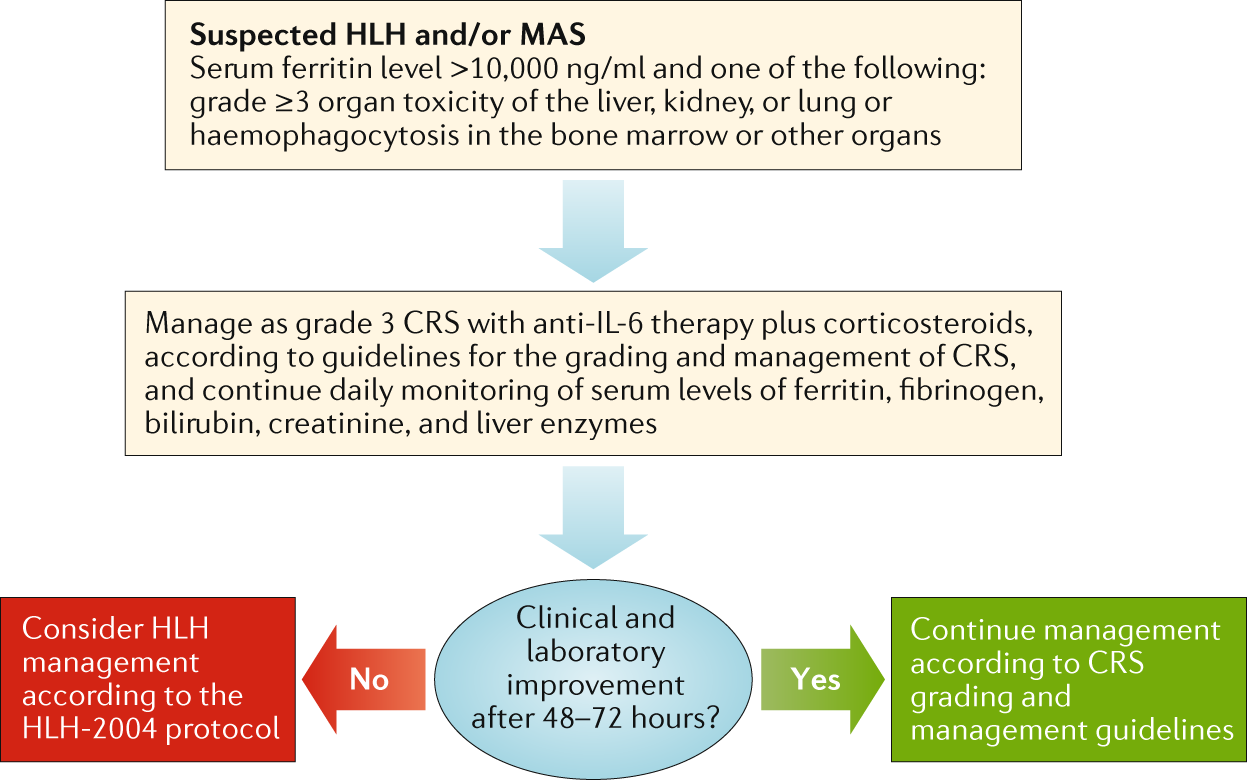
Management guidelines for paediatric patients receiving chimeric antigen receptor T cell therapy | Nature Reviews Clinical Oncology

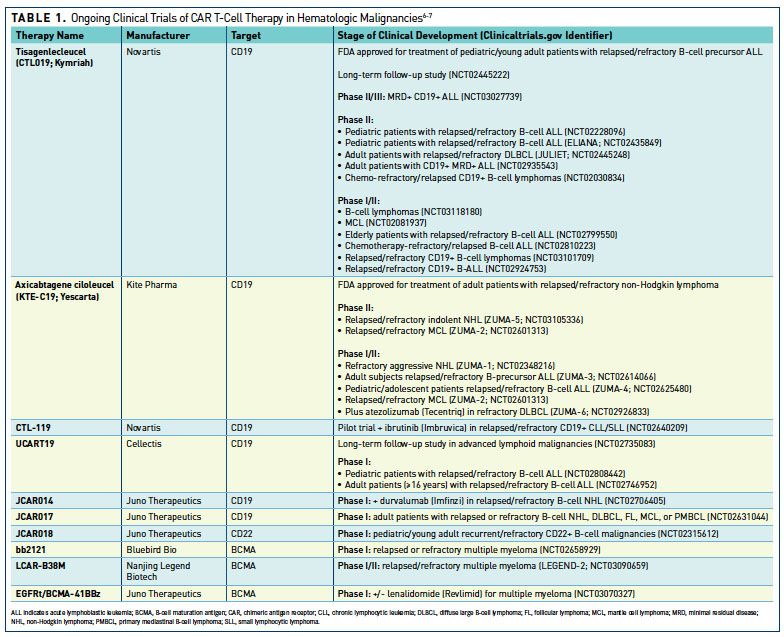
FDA Advisory Committee Recommends Approval of CAR-T Cell Therapy and Two New Biosimilars | Biosimilars Law Bulletin
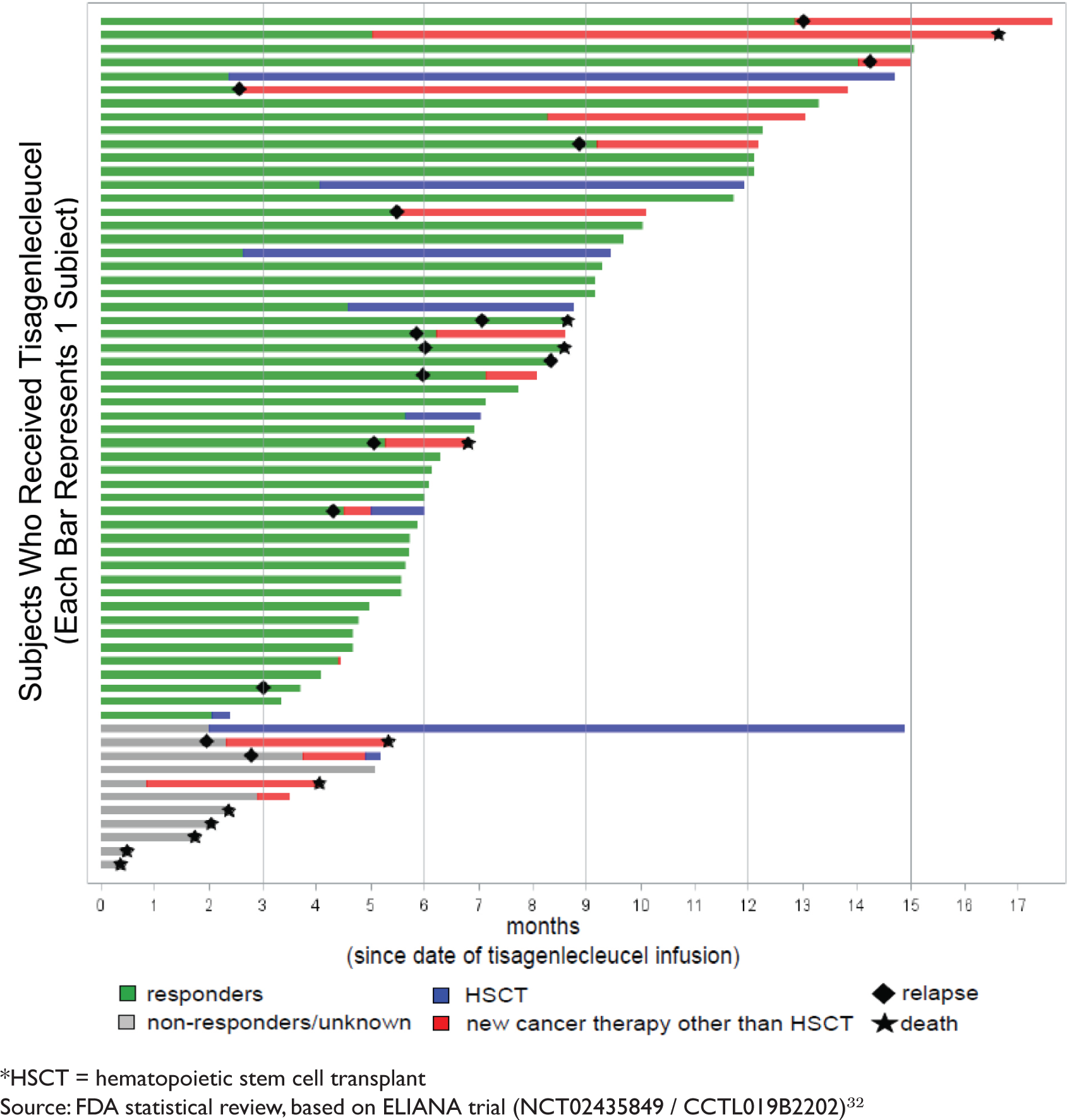
A New Approach to Treat Childhood Leukemia: Novartis' CAR-T Therapy | Journal of Law, Medicine & Ethics | Cambridge Core